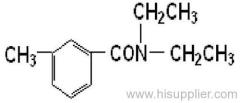
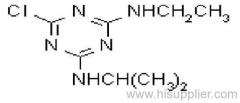
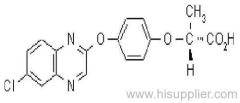
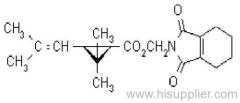
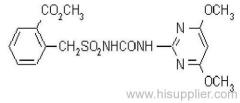|
Shanghai Skyblue Chemical Co., Ltd.
|
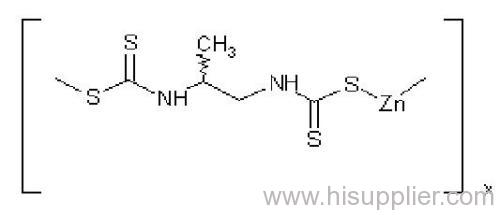
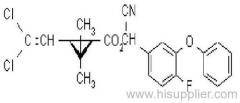
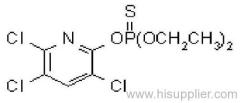
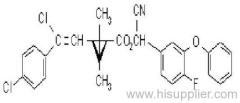
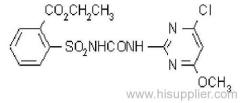
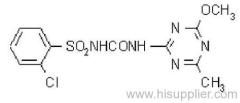
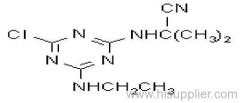
Propineb
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Basic foliar fungicide with protective action. Conidia or germinating conidia are killed by contact.
Common name: propineb; propinèbe
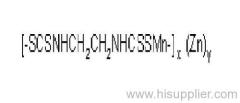
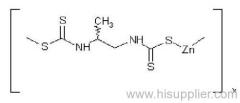
IUPAC name: polymeric zinc propylenebis(dithiocarbamate)
Chemical Abstracts name: [[(1-methyl-1,2-ethanediyl)bis[carbamodithioato]](2-)]zinc homopolymer
CAS RN: [12071-83-9] monomer; [9016-72-2] (formerly [31530-30-0]) homopolymer
PHYSICAL CHEMISTRY
Mol. wt.: 289.8 (theoretical monomer). M.f.: (C5H8N2S4Zn)x Form: White powder, with a slight, characteristic smell. M.p.: Decomposes above 150 ºC. V.p.: <1 mPa (20 ºC). KOW: logP = -0.26 (PTU, main metabolite) (20 ºC). S.g./density: 1.813 g/ml (23 ºC). Solubility: In water 0.01 g/l (20 ºC). In toluene, hexane, dichloromethane <0.1 g/l.; in DMF + DMSO >200 g/l. Stability: Stable when dry. Decomposed by moisture, and in acidic and alkaline media; DT50 (22 ºC) (est.) 1 d (pH 4), c. 1 d (pH 7), >2 d (pH 9).
APPLICATIONS
Biochemistry: Non-specific, multi-site fungicide.
Mode of action: Basic foliar fungicide with protective action. Conidia or germinating conidia are killed by contact.
Uses: Control of downy mildew, black rots, red fire disease, and grey mould on vines; scab and brown rot on apples and pears; leaf spot diseases on stone fruit; Alternaria and Phytophthora blights, downy mildew, Septoria leaf spot, and leaf mould in tomatoes; Phytophthora and Alternaria blight of potatoes; downy mildew (blue mould) on tobacco; rusts and leaf spot diseases on ornamentals; rusts, leaf spot diseases and downy mildews on vegetables. Also used on citrus fruit and berry fruit, rice and tea.
Phytotoxicity: Non-phytotoxic when used as recommended, also in young sensitive growing stages.
Formulation types: DP; WG; WP.
Compatibility: Compatible with most other powder-formulated pesticides. Not stable when combined with alkaline materials.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for rats >5000 mg/kg.
Skin and eye: Acute percutaneous LD50 for rats >5000 mg/kg. Not irritating to eyes and skin (rabbits).
Inhalation: LC50 (4 h) for rats >0.7 mg/l air (aerosol).
NOEL: (2 y) for rats 50, mice 800, dogs 1000 mg/kg diet.
ADI: 0.007 mg/kg b.w.
Toxicity class: WHO (a.i.) III (Table 5); EPA (formulation) IV
ECOTOXICOLOGY
Birds: LD50 for Japanese quail >5000 mg/kg.
Fish: LC50 (96 h) for rainbow trout 1.9, golden orfe 133 mg/l.
Daphnia: LC50 (48 h) 4.7 mg/l.
Algae: ErC50 (96 h) 2.7 mg/l.
Bees: Not toxic to bees; LD50 (oral; 70 WP, 70 WG) >100 ug/bee.
Worms: LD50 (14 d) for 70 WP and 70 WG >1000 ug/kg dry substrate.
Other beneficial spp.: Effects on non-target insects are unlikely; only predatory mites are sensitive. Under field conditions, only moderate effects were observed.
ENVIRONMENTAL FATE
Animals: Elimination is quick; almost 91% is excreted within 48 h in the urine and faeces, and 7% with exhaled air.
Plants: Residues of the applied compound including its metabolite propylenethiourea (PTU) were found mainly on the plant surface. Only the metabolites propyleneurea and 4-methyl-imidazoline were taken up into the plant, in very small amounts. PTU has to be considered as a relevant residue with regard to its toxicological properties.
Soil/Environment: Degradation is very rapid. Propineb can be classified as not mobile in soils.