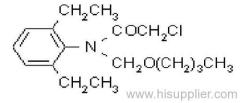|
Shanghai Skyblue Chemical Co., Ltd.
|
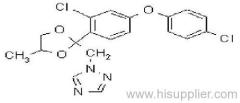
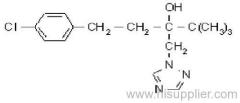
Acetochlor
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Selective herbicide, absorbed mainly by the shoots and secondarily by the roots of germinating plants.
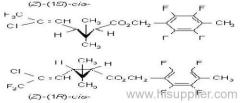
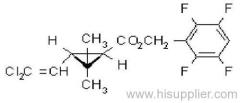
Common name: acetochlor; acétochlore
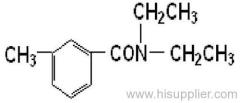
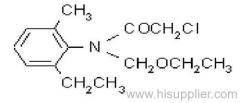
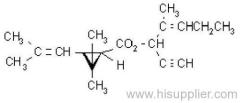
IUPAC name: 2-chloro-N-ethoxymethyl-6'-ethylaceto-o-toluidide
Chemical Abstracts name: 2-chloro-N-(ethoxymethyl)-N-(2-ethyl-6-methylphenyl)acetamide
CAS RN: [34256-82-1]
PHYSICAL CHEMISTRY
Composition: Tech. is 94% pure. Mol. wt.: 269.8; M.f.: C14H20ClNO2; Form: Clear viscous liquid; (tech. is a wine red to yellow or amber oil). M.p.: 10.6 °C. B.p.: 172 ºC/5 mmHg. KOW: logP = 4.14; Henry: 3.83x10-1 Pa m3 mol-1 (calc.) S.g./density: 1.1221 (20 °C). Solubility: In water 223 mg/l (25 ºC). Soluble in diethyl ether, acetone, benzene, chloroform, ethanol, ethyl acetate, and toluene. Stability: Stable for over 2 years at 20 ºC (EC formulation). F.p.: 160 °C (Tag closed cup).
APPLICATIONS
Biochemistry: Inhibits cell division by blocking protein synthesis. Maize tolerance of chloroacetamides is due mainly to conjugation with glutathione; P450 metabolism may also be a factor.
Mode of action: Selective herbicide, absorbed mainly by the shoots and secondarily by the roots of germinating plants.
Uses: Used pre-emergence or pre-plant to control annual grasses, certain annual broad-leaved weeds and yellow nutsedge in maize (at 3 kg/ha), peanuts, soya beans, cotton, potatoes and sugar cane.
Formulation types: CS; EC.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for rats 2148 mg/kg.
Skin and eye: Acute percutaneous LD50 for rabbits 4166 mg/kg. Contact sensitisation reactions observed in guinea pigs. Practically non-irritating to eyes and skin (rabbits).
Inhalation: LC50 (4 h) for rats >3.0 mg/l air.
NOEL: (2 y) for rats 10 mg/kg b.w. daily; (1 y) for dogs 12 mg/kg b.w. daily.
ADI: 0.01 mg/kg b.w.
Toxicity class: WHO (a.i.) III
EC hazard: Xn; R20| Xi; R37/38| R43| N; R50, R53
ECOTOXICOLOGY
Birds: Acute oral LD50 for bobwhite quail 1260 mg/kg. LC50 (5 d) for quail and mallard ducks >5620 mg/kg.
Fish: LC50 (96 h) for rainbow trout 0.36, bluegill sunfish 1.5 mg/l.
Daphnia: LC50 (48 h) 9 mg/l.
Bees: LD50 (24 h) (contact) >200 ug/bee; (oral) >100 ug/bee.
Worms: LC50 (14 d) 211 mg/kg.
ENVIRONMENTAL FATE
Animals: The primary routes of metabolism are glutathione conjugation and metabolism by cytochrome P450.
Plants: In maize and soya beans, rapidly absorbed and metabolised in the germinating plant. In maize, the first metabolite is glutathione, and in soya beans homoglutathione.
Soil/Environment: Adsorbed by soil, with little leaching. Microbial degradation accounts for most loss from soil; DT50 8-18 d.