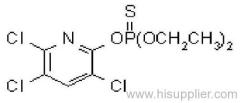
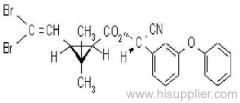
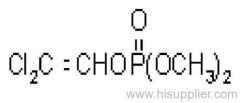
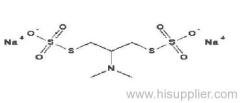
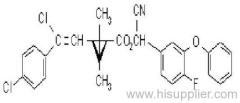
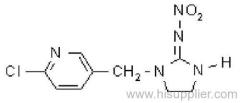
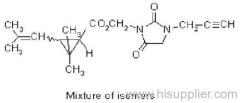
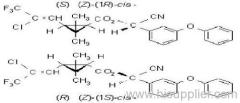
|
Shanghai Skyblue Chemical Co., Ltd.
|
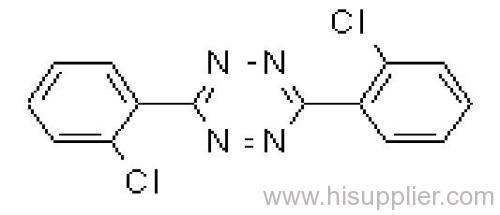
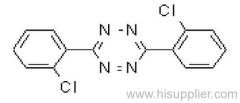
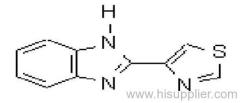
Clofentezine
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Specific acaricide with contact action, and long residual activity. Inhibits embryo development.
Common name: clofentezine
IUPAC name: 3,6-bis(2-chlorophenyl)-1,2,4,5-tetrazine
Chemical Abstracts name: 3,6-bis(2-chlorophenyl)-1,2,4,5-tetrazine
CAS RN: [74115-24-5]
PHYSICAL CHEMISTRY
Mol. wt.: 303.1; M.f.: C14H8Cl2N4; Form Magenta crystals. M.p.: 182.3 ºC; V.p.: 1.3x10-4 mPa (25 ºC) (gas saturation and glc). KOW: logP = 4.1 (25 ºC). Henry: 1.97x10-4 Pa m3 mol-1 (calc.) S.g./density: 1.51 (20 °C). Solubility: In water 2.5 ug/l (pH 5, 22 ºC). In dichloromethane 37, acetone 9.3, hexane 1, ethanol 0.5 (all in g/l, 25 ºC). Stability: The a.i. and formulated products are stable to light, heat and air; on hydrolysis (22 ºC), DT50 248 h (pH 5), 34 h (pH 7), 4 h (pH 9). F.p.: Low flammability.
APPLICATIONS
Mode of action: Specific acaricide with contact action, and long residual activity. Inhibits embryo development.
Uses: Control of eggs and young motile stages (but not adults) of Panonychus ulmi and Tetranychus spp. on pome fruit and stone fruit (20 g/hl), citrus fruit (12.5-20 g/hl), nuts, vines (20 g/hl), hops, strawberries (200-300 g/ha), cucurbits (100-400 g/ha), cotton (150-250 g/ha), and ornamentals (20-30 g/hl). Has no effect on predatory mites or beneficial insect species.
Phytotoxicity: May cause slight injury to glasshouse roses. May cause a slight pink deposit on petals of white or pale flowers.
Formulation types: SC; WP.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for rats >5200 mg/kg.
Skin and eye: Acute percutaneous LD50 for rats >2100 mg/kg. Mild eye and skin irritant. Inhalation LC50 (4 h) for rats >9 mg/l air.
NOEL: (2 y) for rats 40 mg/kg diet; (1 y) for dogs 50 mg/kg diet.
ADI: 0.02 mg/kg b.w.
Toxicity class: WHO (a.i.) III (Table 5); EPA (formulation) III
ECOTOXICOLOGY
Birds: Acute oral LD50 for mallard ducks >3000, bobwhite quail >7500 mg/kg. Dietary LC50 (8 d) for mallard ducks and bobwhite quail >2000 mg/kg diet.
Fish: LC50 (96 h) for rainbow trout >0.015, bluegill sunfish >0.25 mg/l (limits of solubility).
Daphnia: LC50 (48 h) >1.45 ug/l (limit of solubility).
Algae: Not toxic to Scenedesmus panonicus up to limit of solubility.
Bees: Acute LD50 (oral) >20 ug/bee; LC50 (contact) >1500 ppm.
ENVIRONMENTAL FATE
Animals: In mammals, undergoes metabolism by hydroxylation and exchange of the chlorine atoms on the rings for methylthio groups. Following oral administration, excretion occurs within 24-48 hours in the urine and faeces.
Plants: In metabolism studies, unchanged clofentezine was the major extractable residue. Trace amounts (4%) of 2-chlorobenzonitrile, the major photodegradation product, were also detected.
Soil/Environment: In soil, the major degradation route leads to 2-chlorobenzoic acid, and finally to CO2. DT50 in soil 65-85 d (15 ºC), 28-56 d (25 ºC), depending upon soil type. However, in laboratory studies, no leaching occurs. In water, 2-chlorobenzonitrile is the major product formed by hydrolysis and photodegradation, with smaller amounts of other compounds. Low solubility in water makes determination of soil adsorption constants difficult.