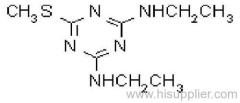
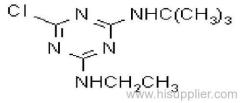
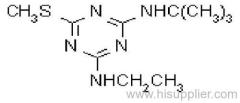
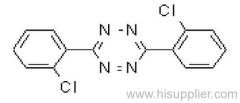
|
Shanghai Skyblue Chemical Co., Ltd.
|
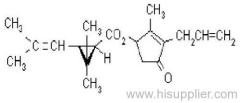
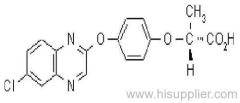
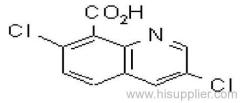
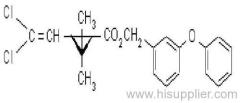
Quizalofop-p-ethyl
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Systemic herbicide, absorbed from the leaf surface, with translocation throughout the plant, moving in both the xylem and phloem, and accumulating
quizalofop-P
Common name: quizalofop-P
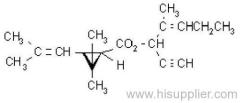
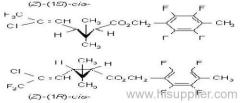
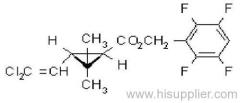
IUPAC name: (R)-2-[4-(6-chloroquinoxalin-2-yloxy)phenoxy]propionic acid
Chemical Abstracts name: (R)-2-[4-[(6-chloro-2-quinoxalinyl)oxy]phenoxy]propanoic acid
CAS RN: [94051-08-8]
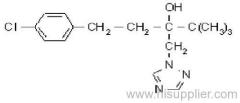
quizalofop-P-ethyl
Common name: quizalofop-P-ethyl
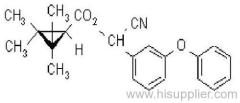
IUPAC name: ethyl (R)-2-[4-(6-chloroquinoxalin-2-yloxy)phenoxy]propionate
CAS RN: [100646-51-3]
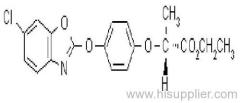
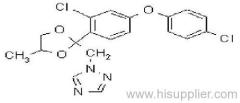
quizalofop-P-tefuryl
Common name: quizalofop-P-tefuryl
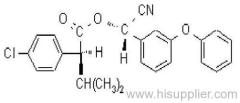
IUPAC name: (tetrahydrofurfuryl-(R)-2-[4-(6-chloroquinoxalin-2-yloxy)phenoxy]propionate
Chemical Abstracts name: (RS)-2-tetrahydrofuranylmethyl (R)-2-[4-(6-chloro-2-quinoxalinyloxy)phenoxy]propanoate
CAS RN: [119738-06-6]
PHYSICAL CHEMISTRY
quizalofop-P
Mol. wt.: 344.8; M.f.: C17H13ClN2O4
quizalofop-P-ethyl
Mol. wt.: 372.8; M.f.: C19H17ClN2O4; Form: White, crystalline, odourless solid. M.p.: 76.1-77.1 ºC. B.p.: 220 ºC/26.6 Pa V.p.: 1.1x10-4 mPa (20 ºC). KOW: logP = 4.66 S.g./density: 1.36 g/cm3 Solubility: In water 0.61 mg/l (20 ºC). In acetone, ethyl acetate and xylene >250, 1,2-dichloroethane >1000 (all in g/l, 22-23 °C); in methanol 34.87, n-heptane 7.168 (both in g/l, 20 °C). Stability: Stable in neutral and acidic media, but unstable in alkaline media; DT50 <1 d (pH 9). Stable at high temperatures and in organic solvents. Specific rotation: [a]D20 +35.9?(20 ºC).
quizalofop-P-tefuryl
Mol. wt.: 428.9; M.f.: C22H21ClN2O5 Form: Thick yellow liquid, which can crystallise upon standing at room temperature. M.p.: 59-68 ºC; (tech. is a liquid). V.p.: 7.9x10-3 mPa (25 ºC, gas saturation method). KOW: logP = 4.32 (25 ºC). Henry: 8.47x10-4 Pa m3 mol-1 (calc.) S.g./density: 1283 kg/m3 (21.5 ºC). Solubility: In water 4 mg/l (25 ºC). In toluene 652, hexane 12, methanol 64 (all in g/l). Stability: Stable for 14 d at 55 ºC; stable in package for 1 y at 25 ºC. Stable 7 d in sunlight. In water, DT50 (22 ºC) 82 d, 8.1 d (pH 5), 431 min (pH 9). pKa: 1.25 (25 ºC). F.p.: >110 ºC (Pensky-Martens).
APPLICATIONS
quizalofop-P-ethyl
Biochemistry: Acetyl CoA carboxylase inhibitor; inhibition of fatty acid biosynthesis.
Mode of action: Systemic herbicide, absorbed from the leaf surface, with translocation throughout the plant, moving in both the xylem and phloem, and accumulating in the meristematic tissue.
Uses: Selective post-emergence control of annual and perennial grass weeds in potatoes, soya beans, sugar beet, peanuts, oilseed rape, sunflowers, vegetables, cotton, and flax.
Phytotoxicity: Most non-graminaceous crops are tolerant.
Formulation types: EC; SC.
Compatibility: Can be used in combination with post-emergence broad-leaved herbicides.
quizalofop-P-tefuryl
Mode of action: Systemic herbicide, absorbed from the leaf surface, with translocation throughout the plant, moving in both the xylem and phloem, and accumulating in the meristematic tissue.
Uses: Control of annual grasses such as Avena fatua and Alopecurus myosuroides, and perennial grasses such as Sorghum halepense and Elymus repens, in potatoes, flax, oilseed rape, peas, sugar beet, cotton, and soya beans.
Formulation types: EC.
Compatibility: Certain post-emergence broad-leaved herbicides are incompatible with quizalofop-P-tefuryl.
MAMMALIAN TOXICOLOGY
quizalofop-P-ethyl
Oral: Acute oral LD50 for male rats 1210, female rats 1182, male mice 1753, female mice 1805 mg/kg.
NOEL: (90 d) for rats 7.7 mg/kg daily.
Toxicity class: EPA (formulation) II (5EC)
quizalofop-P-tefuryl
Oral: Acute oral LD50 for rats 1012 mg/kg.
Skin and eye: Acute percutaneous LD50 for rabbits >2000 mg/kg. Mild eye irritation; non-irritating to skin (rabbits). Non-sensitising to skin (guinea pigs).
Inhalation: LC50 (4 h) for rats >4.8 mg/l (12% EC).
NOEL: In chronic oncogenic feeding studies, NOEL for rats 1.25, dogs 19 mg/kg b.w. daily.
ADI: 0.01 mg/kg.
Toxicity class: WHO (a.i.) II; EPA (formulation) III
ECOTOXICOLOGY
quizalofop-P-ethyl
Birds: Acute oral LD50 for mallard ducks and bobwhite quail >2000 mg/kg.
Fish: LC50 (96 h) for rainbow trout >0.5 mg/l.
Daphnia: LC50 (48 h) 0.29 mg/l.
Algae: Assumed similar to racemate.
Bees: Assumed similar to racemate.
Worms: LC50 >1000 mg/kg.
quizalofop-P-tefuryl
Birds: Acute oral LD50 for bobwhite quail and mallard ducks >2150 mg/kg. LC50 (8 d) for bobwhite quail and mallard ducks >5000 ppm.
Fish: LC50 (96 h) for trout 0.51, sunfish 0.23 mg/l.
Daphnia: LC50 (48 h) >1.5 mg/l.
Algae: LC50 (5 d) for Selenastrum >1.9 mg/l.
Bees: LD50 >100 ug/bee.
ENVIRONMENTAL FATE
quizalofop-P-ethyl
Animals: The degradation pattern is the same as that for quizalofop-ethyl.
Plants: The degradation pattern is the same as that for quizalofop-ethyl.
quizalofop-P-tefuryl
Animals: Main metabolite is quizalofop acid.
Plants: Main metabolite is quizalofop acid.
Soil/Environment: Aerobic DT50 4.7 h (sandy loam). Adsorption Kd 8.69, Koc 477 (both in sandy loam).
Didn't find what you're looking for?
Post Buying Lead or contact
HiSupplier Customer Service Center
for help!