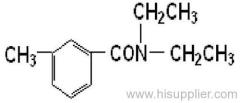
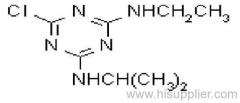
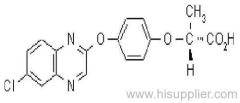
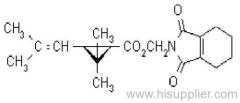
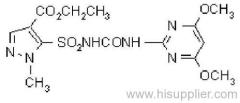
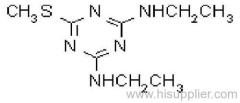
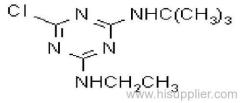
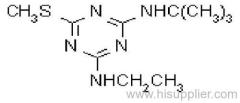
|
Shanghai Skyblue Chemical Co., Ltd.
|
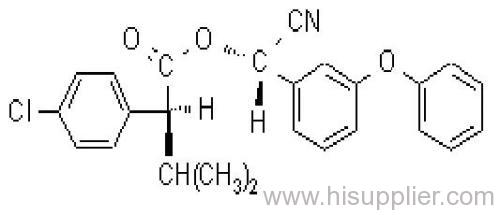
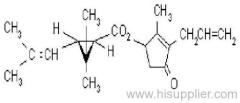
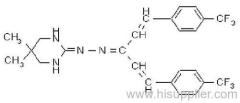
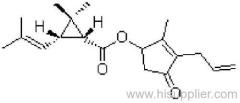
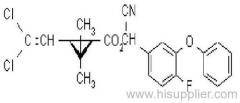
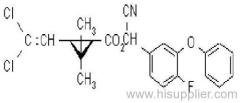
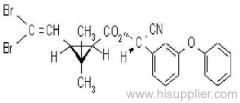
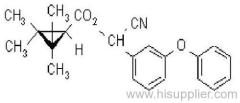
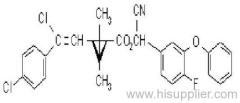
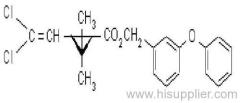
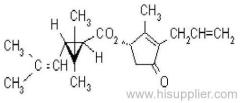
Es-fenvalerate
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Insecticide with contact and stomach action.
Common name: esfenvalerate
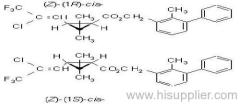
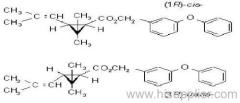
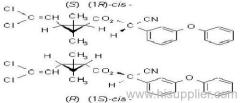
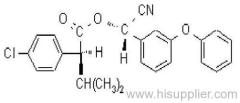
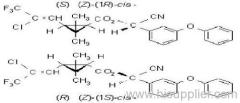
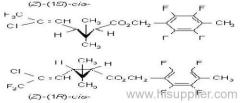
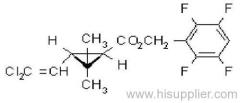
IUPAC name: (S)-a-cyano-3-phenoxybenzyl (S)-2-(4-chlorophenyl)-3-methylbutyrate
Chemical Abstracts name: [S-(R*,R*)]-cyano(3-phenoxyphenyl)methyl 4-chloro-2-(1-methylethyl)benzeneacetate
Other names: fenvalerate-U
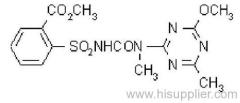
CAS RN: [66230-04-4]
PHYSICAL CHEMISTRY
Composition: Tech. is 95% total isomers and 75% resolved (S,S)- isomers. Mol. wt.: 419.9; M.f.: C25H22ClNO3; Form: Colourless crystals; (tech., yellow-brown viscous liquid or solid at 23 ºC). M.p.: 59.0-60.2 ºC; (tech., 43.3-54 °C). B.p.: 151-167 ºC (tech.) V.p.: 2x10-4 mPa (25 ºC). KOW: logP = 6.22 (25 ºC). Henry: 4.20x10-2 Pa m3 mol-1 (calc.) S.g./density: 1.26 (4-26 ºC) Solubility: In water 0.002 mg/l (25 ºC). In xylene, acetone, chloroform, ethyl acetate, dimethylformamide, dimethyl sulfoxide >600, hexane 10-50, methanol 70-100 (all in g/kg, 25 ºC). Stability: Relatively stable to heat and light. Stable to hydrolysis at pH 5, 7 and 9 (25 °C). Specific rotation: [a]D25 -15.0?(c 2.0 in methanol). F.p.: 256 °C (Pensky-Martens).
APPLICATIONS
Biochemistry: Voltage dependent sodium channel agonist.
Mode of action: Insecticide with contact and stomach action.
Uses: A potent contact and ingested insecticide with a very broad range of activity, especially effective against Coleoptera, Diptera, Hemiptera, Lepidoptera and Orthoptera on cotton, fruit, vegetables and other crops, at 5-25 g a.i./ha. It is effective against strains resistant to organochlorine, organophosphorus and carbamate insecticides.
Phytotoxicity: Some injury has been noted on crucifers, cucumbers, aubergines, tomatoes, pears and mandarin oranges.
Formulation types EC; SC; UL.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for rats 75-88 mg/kg.
Skin and eye: Acute percutaneous LD50 for rats >5000, rabbits >2000 mg/kg. Slight skin irritant; mild eye irritant. Not a skin sensitiser.
NOEL: No effect on sub-chronic studies at 2 mg/kg daily.
ADI: 0.02 mg/kg.
Other: Acute LD50 values vary with the vehicle, concentration, route and species, etc.; values reported sometimes differ markedly. No carcinogenic, developmental or reproductive toxicity in animal tests.
Toxicity class: WHO (a.i.) II; EPA (formulation) II
EC hazard: T; R23/25| R43| N; R50, R53
ECOTOXICOLOGY
Birds: Acute oral LD50 for bobwhite quail 381 mg/kg. LC50 (8 d) for bobwhite quail >5620, mallard ducks 5247 ppm.
Fish: Extremely toxic to aquatic animals. LC50 (96 h) for fathead minnows 0.690, bluegill sunfish 0.26, rainbow trout 0.26 ug/l.
Daphnia: LC50 (48 h) 0.24 ug/l.
Bees: LD50 (contact) 0.017 ug/bee.
ENVIRONMENTAL FATE
Animals: Rapid metabolism and elimination occurs in rats and other animals. Primary metabolism involves hydroxylation of 2'- and 4'- hydroxyl moieties, ester cleavage, hydroxylation and oxidation of the alcohol derivatives, oxidation of the cyano moiety and conjugation of the acidic metabolites with sulfate, glycine and glucuronic acid.
Plants: The major metabolite was decarboxylated fenvalerate. Ester cleavage, hydration of the cyano group to carboxamide and carboxylic acid, hydroxylation of the 2'- and 4'- phenoxy positions, conversion of the alcohol moiety to 3-phenoxybenzyl alcohol and 3-phenoxybenzoic acid, and conjugation of the resulting carboxylic acids and alcohols with sugars, also occur.
Soil/Environment: In sand (0.38% o.m.), Kd (25 ºC) 4.4; in sandy loam (pH 7.3, 1.1% o.m.), Kd (25 ºC) 6.4, DT50 88 d; in silty loam (pH 5.3, 2.0% o.m.), Kd (25 ºC) 71, DT50 114 d; in clay loam (pH 5.7, 0.2% o.m.), DT50 287 d; in clay loam (pH 6.4, 1.5% o.m.), Kd (25 ºC) 105. Koc 5300.