|
Shanghai Skyblue Chemical Co., Ltd.
|
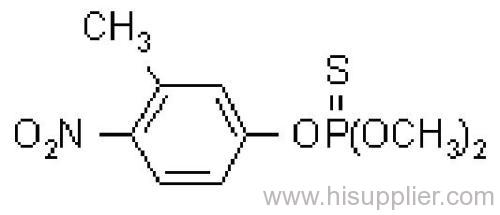
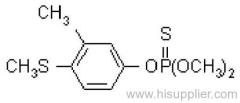
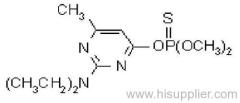
Fenitrothion
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Non-systemic insecticide with contact and stomach action.
Common name: fenitrothion
IUPAC name: O,O-dimethyl O-4-nitro-m-tolyl phosphorothioate
Chemical Abstracts name: O,O-dimethyl O-(3-methyl-4-nitrophenyl) phosphorothioate
CAS RN: [122-14-5]
PHYSICAL CHEMISTRY
Mol. wt.: 277.2; M.f.: C9H12NO5PS; Form: Yellow-brown liquid, with a faint characteristic odour. M.p.: 0.3 °C; B.p.: 140-145 ºC/0.1 mmHg (decomp.) V.p.: 18 mPa (20 ºC). KOW: logP = 3.43 (20 ºC) S.g./density: 1.328 (25 ºC) Solubility: In water 14 mg/l (30 ºC). Readily soluble in alcohols, esters, ketones, aromatic hydrocarbons, and chlorinated hydrocarbons. In hexane 24, isopropanol 138 (both in g/l, 20 ºC). Stability: Relatively stable to hydrolysis under normal conditions: DT50 (est.) 108.8 d (pH 4), 84.3 d (pH 7), 75 d (pH 9) (22 ºC). F.p.: 157 °C.
APPLICATIONS
Biochemistry: Cholinesterase inhibitor.
Mode of action: Non-systemic insecticide with contact and stomach action.
Uses: Control of chewing, sucking, and boring insects in cereals, soft fruit, tropical fruit, vines, rice, sugar cane, vegetables, turf, and forestry. Also used as a public health insecticide for control of household insects (flies, cockroaches, and other insects) by application to breeding sites; for control of flies in animal houses; for control of stored product insect pests; for control of mosquito larvae (as a vector control agent for malaria); and for control of locusts.
Phytotoxicity: Non-phytotoxic when used as recommended. Cotton, brassicas, and some fruits may be injured by high rates of application. Russetting is possible with some apple varieties.
Formulation types: AE; DP; EC; GR; UL; WP.
Compatibility: Incompatible with alkaline compounds.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for male rats c. 1700, female rats 1720 mg/kg.
Skin and eye: Acute percutaneous LD50 for male rats c. 810, female rats 840 mg/kg. Not a skin or eye irritant (rabbits).
Inhalation: LC50 (4 h) for rats >2210 mg/m3 (aerosol).
NOEL: (2 y) for rats and mice 10 mg/kg diet; (1 y) for dogs 50 mg/kg diet.
ADI: 0.005 mg/kg b.w.
Other: Not carcinogenic, mutagenic, embryotoxic or teratogenic.
Toxicity class: WHO (a.i.) II; EPA (formulation) II
EC hazard: Xn; R22| N; R50, R53
ECOTOXICOLOGY
Birds: Acute oral LD50 for quail 23.6, mallard ducks 1190 mg/kg.
Fish: LC50 (48 h) for carp 4.1 mg/l. LC50 (96 h) for bluegill sunfish 2.5, rainbow trout 1.3 mg/l.
Daphnia: EC50 (48 h) 0.0086 mg/l.
Algae: EC50 (96 h) for Selenastrum capricornutum 1.3 mg/l.
Bees: Toxic to bees.
Other beneficial spp.: Highly toxic to non-target arthropods.
ENVIRONMENTAL FATE
Animals: Fenitrothion is rapidly excreted in the urine and faeces. After 3 d, c. 90% has been excreted by rats, mice and rabbits. The most important metabolites are dimethylfenitrooxon and 3-methyl-4-nitrophenol.
Plants: Fenitrothion applied in the forest (balsam fir and spruce foliage) degraded, with DT50 4 d; 70-85% is degraded within 2 weeks. Similar results are obtained in conifer foliage and cocoa trees. Major metabolites are 3-methyl-4-nitrophenol, the oxygen analogue and their decomposition products desmethylfenitrothion, dimethylphosphorothionic acid and phosphorothionic acid.
Soil/Environment: DT50 12-28 d under upland conditions, 4-20 d under submerged conditions. The major metabolites under upland conditions are 3-methyl-4-nitrophenol and CO2, whereas, under submerged conditions, the major decomposition product is aminofenitrothion.