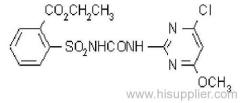
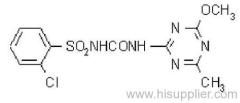
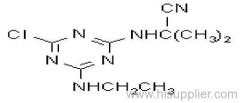
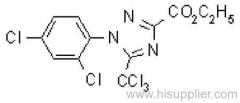
|
Shanghai Skyblue Chemical Co., Ltd.
|
Gold Index: 3880
You are here: home > Fenpropathrin
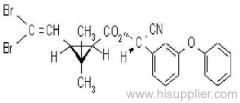
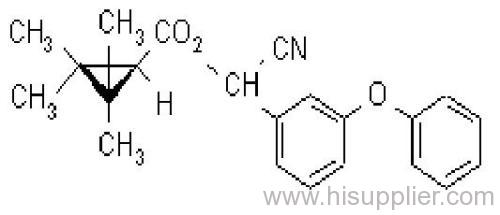
Fenpropathrin
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Model No.:
64257-84-7
Acaricide and insecticide with repellent, and contact and stomach action.
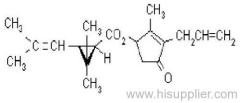
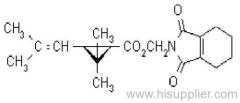
Common name: fenpropathrin; fenpropathrine
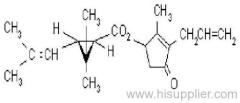
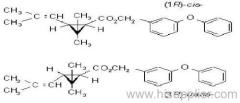
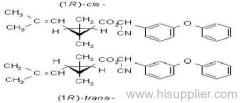
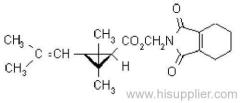
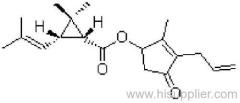
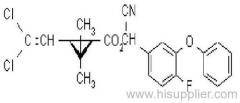
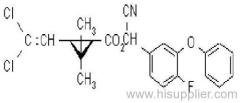
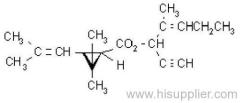
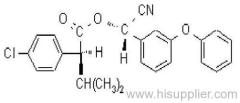
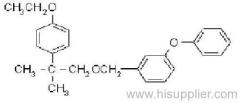
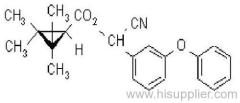
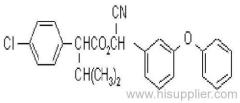
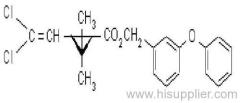
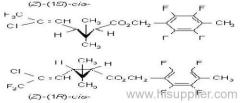
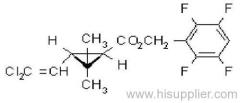
IUPAC name: (RS)-a-cyano-3-phenoxybenzyl 2,2,3,3-tetramethylcyclopropanecarboxylate
Chemical Abstracts name: cyano(3-phenoxyphenyl)methyl 2,2,3,3-tetramethylcyclopropanecarboxylate
CAS RN: [64257-84-7] racemate; [39515-41-8] unstated stereochemistry
PHYSICAL CHEMISTRY
Mol. wt.: 349.4; M.f.: C22H23NO3; Form: Yellow-brown solid (tech.). M.p.: 45-50 ºC; V.p.: 0.730 mPa (20 ºC); KOW: logP = 6 (20 ºC); S.g./density: 1.15 (25 ºC). Solubility: In water 14.1 ug/l (25 ºC). In xylene, cyclohexanone 1000, methanol 337 (all in g/kg, 25 ºC). Stability: Decomposed in alkaline solutions. Exposure to light and air leads to oxidation and loss of activity.
APPLICATIONS
Mode of action: Acaricide and insecticide with repellent, and contact and stomach action.
Uses: Control of many species of mites (except rust mites) and insects (e.g. whitefly, lepidopterous larvae, leaf miners, leafworms, bollworms, etc.) on pome fruit, citrus fruit, vines, hops, vegetables, ornamentals (including ornamental trees), cotton, field crops, and glasshouse crops (cucurbits, tomatoes, ornamentals, etc.).
Formulation types: EC; SC; UL; WP.
Compatibility: Incompatible with alkaline materials.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for male rats 70.6, female rats 66.7 mg/kg (in corn oil).
Skin and eye: Acute percutaneous LD50 for male rats 1000, female rats 870, rabbits >2000 mg/kg. Not a skin irritant; mild eye irritant (rabbits). Non-sensitising to skin.
Inhalation: LC50 (4 h) for rats >96 mg/m3.
ADI: 0.03 mg/kg b.w.
Other: Non-mutagenic.
Toxicity class: WHO (a.i.) II; EPA (formulation) II
EC hazard: T+; R26| T; R25| Xn; R21| N; R50, R53
ECOTOXICOLOGY
Birds: Acute oral LD50 for mallard ducks 1089 mg/kg. Dietary LC50 (8 d) for bobwhite quail and mallard ducks >10 000 mg/kg diet.
Fish: LC50 (48 h) for bluegill sunfish 1.95 ug/l.
ENVIRONMENTAL FATE
Soil/Environment: Degraded principally by photolysis, DT50 2.7 w in river water. Duration of activity in soil c. 1-5 d.
Related Search
Find more related products in following catalogs on Hisupplier.com
Company Info
Shanghai Skyblue Chemical Co., Ltd. [China (Mainland)]
Business Type:Manufacturer, Trading Company
Country/Region: China (Mainland)