|
Shanghai Skyblue Chemical Co., Ltd.
|
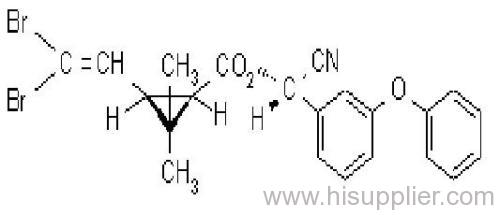
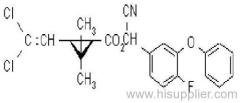
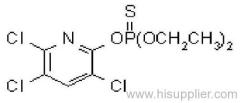
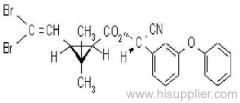
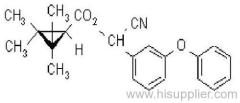
Deltamethrin
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to Basket | Add to My Favorites |
| HiSupplier Escrow |
Product Detail
Non-systemic insecticide with contact and stomach action. Fast-acting.
Common name: deltamethrin; deltaméthrine
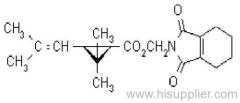
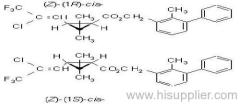
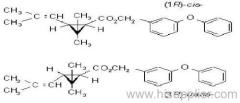
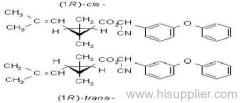
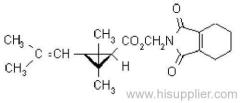
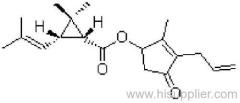
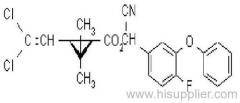
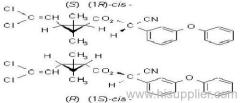
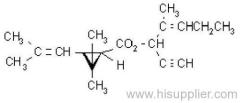
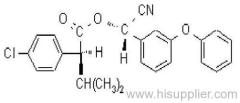
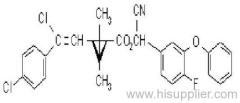
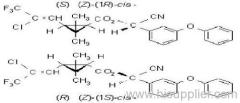
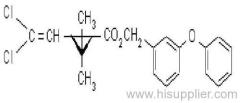
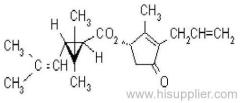
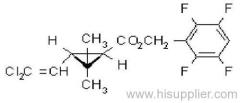
IUPAC name: (S)-a-cyano-3-phenoxybenzyl (1R,3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropanecarboxylate
Roth: (S)-a-cyano-3-phenoxybenzyl (1R)-cis-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropanecarboxylate
Chemical Abstracts name [1R-[1a(S*),3a]]-cyano(3-phenoxyphenyl)methyl 3-(2,2-dibromoethenyl)-2,2-dimethylcyclopropanecarboxylate
Other names: decamethrin* (rejected common name proposal)
CAS RN: [52918-63-5]; [52820-00-5] ((RS)- (1R)-cis- isomer pair)
PHYSICAL CHEMISTRY
Composition: Tech. contains 98% deltamethrin (i.e. the single isomer). Mol. wt.: 505.2; M.f.: C22H19Br2NO3; Form: Colourless crystals. M.p.: 100-102 ºC. V.p.: 1.24x10-5 mPa (25 °C, gas saturation method). KOW: logP = 4.6 (25 ºC). Henry: 3.13x10-2 Pa m3 mol-1 (calc.) S.g./density: Bulk density 0.55 g/cm3 (25 ºC). Solubility: In water <0.2 ug/l (25 ºC). In dioxane 900, cyclohexanone 750, dichloromethane 700, acetone 500, benzene 450, dimethyl sulfoxide 450, xylene 250, ethanol 15, isopropanol 6 (all in g/l, 20 ºC). Stability: Extremely stable on exposure to air. Stable 190 ºC. Under u.v. irradiation and in sunlight, a cis-trans isomerisation, splitting of the ester bond, and loss of bromine occur. More stable in acidic than in alkaline media; DT50 2.5 d (pH 9, 25 ºC).
APPLICATIONS
Biochemistry: Like all pyrethroids, prevents the sodium channels from functioning, so that no transmission of nerve impulses can take place.
Mode of action: Non-systemic insecticide with contact and stomach action. Fast-acting.
Uses: A potent insecticide, effective by contact and ingestion against a wide range of pests. Crop protection uses include: Coleoptera (2.5-7.5 g/ha), Heteroptera (5.0-7.5 g/ha), Homoptera (6.2-12.5 g/ha), Lepidoptera (5.0-21 g/ha) and Thysanoptera (5-10 g/ha) in cereals, citrus, cotton, grapes, maize, oilseed rape, soya beans, top fruit and vegetables. It controls Acrididae (5.0-12.5 g/ha), and is recommended against locusts. Soil surface sprays (2.5-5.0 g/ha) control Noctuidae. It is used against indoor crawling and flying insects (12.5 mg/m2) and pests of stored grain (0.25-0.5 g/t) and timber (Blattodea, Culicidae, Muscidae). Dip or spray (12.5-75 mg/l), and pour-on (0.75 mg/kg b.w.) applications give good control of Muscidae, Tabanidae, Ixodidae and other Acari on cattle, sheep and pigs, etc.
Formulation types DP; EC; EG; EW; GR; HN; PO; SC; SL; TB; UL; WG; WP.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for rats ranges from 135->5000 mg/kg, depending upon carrier and conditions of the study; for dogs >300 mg/kg. Acute oral LD50 for formulations in rats: >2000 mg (of 15 g/l EC)/kg; 445 mg (of 25 g/l EC)/kg; >5000 mg (of 5 g/l UL)/kg; >16 000 mg (of 25 g/kg WP)/kg; >40 000 mg (of 25 g/l SC)/kg.
Skin and eye: Acute percutaneous LD50 for rats and rabbits >2000 mg/kg. Non-irritating to skin; mild eye irritant (rabbits).
Inhalation: LC50 (4 h) for rats 2.2 mg/l air; (1 h) >4.6 mg/l air (micronised).
NOEL: (2 y) for mice 12, rats 1, dogs 1 mg/kg b.w.
ADI: 0.01 mg/kg b.w.
Other: Non-mutagenic and non-teratogenic (mice, rats, rabbits).
Toxicity class: WHO (a.i.) II; EPA (formulation) II
ECOTOXICOLOGY
Birds: Acute oral LD50 for mallard ducks >4640 mg/kg. Dietary LC50 (8 d) for mallard ducks >8039, quail >5620 mg/kg diet. NOEL for reproduction for mallard ducks >70, bobwhite quail >55 mg/kg daily.
Fish: Toxic to fish under laboratory conditions; LC50 (96 h) for rainbow trout 0.91, bluegill sunfish 1.4 ug/l. Not toxic to fish under natural conditions.
Daphnia LC50 (48 h) 3.5 ug/l.
Algae: EC50 (96 h) for algae (Selenastrum capricornutum) >9.1 mg/l. Low LD50 and LC50 values under laboratory conditions do not represent a significant hazard to aquatic fauna in normal field use.
Bees: Toxic to bees, LD50 (oral) 79 ng/bee; (contact) 51 ng/bee. Low LD50 and LC50 values under laboratory conditions do not represent a significant hazard to bees in normal field use.
Worms: LC50 (14 d) for earthworms >1290 mg/kg soil.
ENVIRONMENTAL FATE
Animals: In rats, following oral administration, elimination occurs within 2-4 days. The phenyl ring is hydroxylated, the ester bond hydrolysed, and the acid moiety is eliminated as the glucuronide and glycine conjugates.
Plants: No uptake through leaves and roots - non-systemic compound. No major metabolites, except in oily crops, where trans-deltamethrin is part of the residue definition.
Soil/Environment: In soil, undergoes microbial degradation within 1-2 weeks. Kd 3790-30 000, Koc 4.6x105-1.63x107 cm3/g, confirms strong adsorption by soil colloids and no risk of leaching. DT50 (lab., aerobic) 21-25 d, (anaerobic) 31-36 d. In field, DT50 <23 d. Soil photolysis DT50 9 d. No incidence on soil microflora and nitrogen cycle.