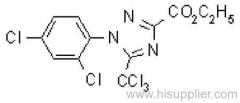
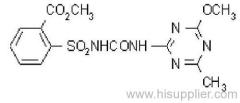
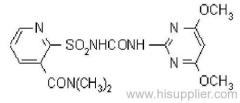
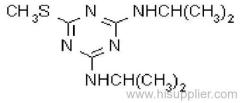
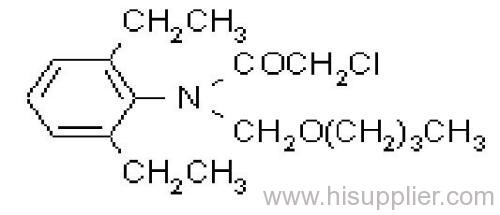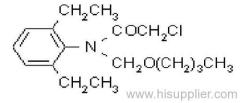|
Shanghai Skyblue Chemical Co., Ltd.
|
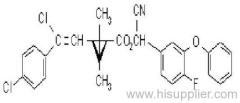
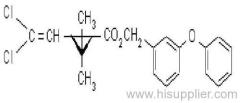
Butachlor
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Selective systemic herbicide, absorbed primarily by the germinating shoots, and secondarily by the roots, with translocation throughout the plant, ...
Common name: butachlor
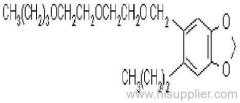
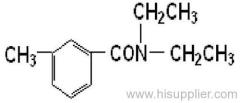
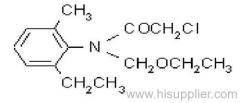
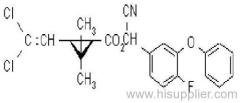
IUPAC name: N-butoxymethyl-2-chloro-2',6'-diethylacetanilide
Chemical Abstracts name: N-(butoxymethyl)-2-chloro-N-(2,6-diethylphenyl)acetamide
CAS RN: [23184-66-9]
PHYSICAL CHEMISTRY
Composition: 92% pure. Mol. wt.: 311.9; M.f.: C17H26ClNO2 Form: Light yellow to purple liquid with a faint, sweet odour. M.p.: -2.8 °C to 1.7 °C. B.p.: 156 ºC/0.5 mmHg. V.p.: 2.4x10-1 mPa (25 °C). Henry: 3.74x10-3 Pa m3 mol-1 (calc.) S.g./density: 1.076 (25 ºC). Solubility: In water 20 mg/l (20 ºC). Soluble in most organic solvents, including diethyl ether, acetone, benzene, ethanol, ethyl acetate, and hexane. Stability: Decomposes at 165 ºC. Stable to u.v. light. Stable indefinitely 45 °C. F.p.: >135 °C (Tag closed cup). Other properties: Viscosity 37 cP (25 °C).
APPLICATIONS
Biochemistry: Inhibits cell division by blocking protein synthesis.
Mode of action: Selective systemic herbicide, absorbed primarily by the germinating shoots, and secondarily by the roots, with translocation throughout the plant, giving higher concentrations in vegetative parts than in reproductive parts.
Uses: Used pre-emergence for the control of annual grasses and certain broad-leaved weeds in rice, both seeded and transplanted. It shows selectivity in barley, cotton, peanuts, sugar beet, wheat and several brassica crops. Effective rates range from 1.0-4.5 kg a.i./ha. Activity is dependent on water availability such as rainfall following treatment, overhead irrigation or applications to standing water as in rice culture.
Phytotoxicity: Non-phytotoxic to rice, cotton, barley, wheat, peanuts, sugar beet, and some brassicas.
Formulation types: EC; GR.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for rats 2000, mice 4747, rabbits >5010 mg/kg.
Skin and eye: Acute percutaneous LD50 for rabbits >13 000 mg/kg. Moderate skin irritant; practically non-irritating to eyes (rabbits). Contact sensitisation reactions observed in guinea pigs.
Inhalation: LC50 (4 h) for rats >3.34 mg/l air.
Other: Oncogenic in rats but not in mice. For detailed toxicology data, please contact Monsanto.
Toxicity class: WHO (a.i.) III (Table 5); EPA (formulation) III
ECOTOXICOLOGY
Birds: Acute oral LD50 for mallard ducks >4640 mg/kg. Dietary LC50 (5 d) for mallard ducks >10 000, bobwhite quail 6597 mg/kg diet.
Fish: LC50 (96 h) for rainbow trout 0.52, bluegill sunfish 0.44, carp 0.32, channel catfish 0.10-0.14, fathead minnow 0.31 mg/l.
Daphnia: LC50 (48 h) 2.4 mg/l.
Other aquatic spp.: LC50 (96 h) for crayfish 26 mg/l.
Bees: LD50 (contact) >100 ug/bee.
ENVIRONMENTAL FATE
Animals: Metabolised to water-soluble metabolites and excreted.
Plants: Rapidly metabolised in plants to water-soluble metabolites, leading eventually to mineralisation.
Soil/Environment: In soil, degradation is principally by microbial activity. Persists for c. 6-10 weeks. Converted in soil or water to water-soluble derivatives, with a slow evolution of CO2.