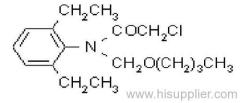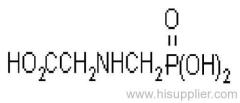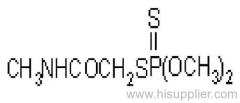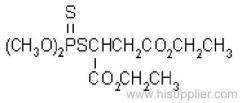|
Shanghai Skyblue Chemical Co., Ltd.
|
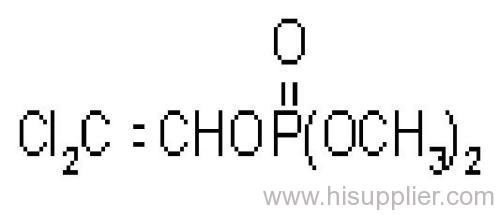
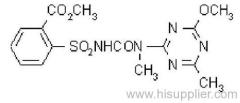
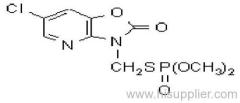
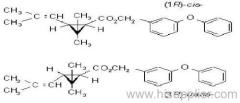
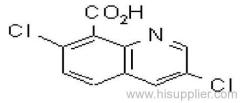
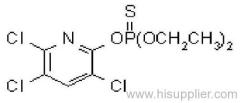
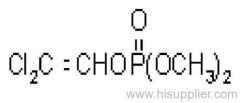
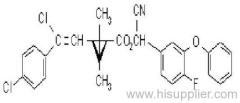
Dichlorvos
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Insecticide and acaricide with respiratory, contact, and stomach action. Gives rapid knockdown.
Common name: dichlorvos; dichlorfos; DDVP
IUPAC name: 2,2-dichlorovinyl dimethyl phosphate
Chemical Abstracts name: 2,2-dichloroethenyl dimethyl phosphate
CAS RN: [62-73-7]
EEC no.: 200-547-7
PHYSICAL CHEMISTRY
Mol. wt.: 221.0; M.f.: C4H7Cl2O4P; Form: Colourless liquid; (tech., colourless-to-amber liquid, with an aromatic odour). B.p.: 234.1 ºC/1x105 Pa (OECD 103); 74 °C/1.3x102 Pa. V.p.: 2.1 103 mPa (25 ºC) (OECD 104). KOW: logP = 1.9 (OECD 117); 1.42 (separate study). Henry: 2.58x10-2 Pa m3 mol-1 S.g./density: 1.425 (20 ºC) (OECD 109). Solubility: In water c. 18 g/l (25 ºC). Completely miscible with aromatic hydrocarbons, chlorinated hydrocarbons and alcohols; moderately soluble in diesel oil, kerosene, isoparaffinic hydrocarbons, and mineral oils. Stability: Stable to heat. Slowly hydrolysed in water and in acidic media, and rapidly hydrolysed by alkalis, to dimethyl hydrogen phosphate and dichloroacetaldehyde; DT50 (est.) 31.9 d (pH 4), 2.9 d (pH 7), 2.0 d (pH 9) (22 ºC). F.p.: >100 °C (DIN 51758); 172 °C (Pensky-Martens closed cup, 1x105 Pa).
APPLICATIONS
Biochemistry: Cholinesterase inhibitor.
Mode of action: Insecticide and acaricide with respiratory, contact, and stomach action. Gives rapid knockdown.
Uses: Control of household and public health insect pests, e.g. flies, mosquitoes, cockroaches, bedbugs, ants, etc.; stored-product pests in warehouses, storerooms, etc.; flies and midges in animal houses; sciarid and phorid flies in mushrooms; sucking and chewing insects, and spider mites in a wide range of crops, including fruit, vines, vegetables, ornamentals, tea, rice, cotton, hops, glasshouse crops, etc. Also used as a veterinary anthelmintic.
Phytotoxicity: Non-phytotoxic when used as directed, except to some varieties of chrysanthemum.
Formulation types: AE; EC; GR; HN; KN; OL; SL; Impregnated strip.
Compatibility: Incompatible with alkaline materials, chinomethionat, and dichlofluanid.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for rats c. 50 mg/kg.
Skin and eye: Acute percutaneous LD50 for rats c. 90 mg/kg. Slight skin and eye irritant (rabbits).
Inhalation: LC50 (4 h) for rats 340 mg/m3; (1 h) for rats 455 mg/m3.
NOEL: (2 y) for rats 10 mg/kg diet.
ADI: 0.004 mg/kg b.w.
Toxicity class: WHO (a.i.) Ib; EPA (formulation) I
EC hazard: T R24/25
ECOTOXICOLOGY
Birds: Acute oral LD50 for bobwhite quail 24 mg/kg. Sub-acute oral LD50 (8 d) for Japanese quail 300 mg/kg.
Fish: LC50 (96 h) for rainbow trout 200, golden orfe 450 ug/l (both 500 EC).
Daphnia: LC50 (48 h) 0.19 ug/l.
Algae: EC50 (5 d) for Scenedesmus subspicatus 52.8 mg/l.
Bees: Acute oral LD50 0.29 ug/bee.
ENVIRONMENTAL FATE
Animals: In mammals, following oral administration, rapidly degraded in the liver by hydrolysis and O-demethylation, with a half-life of c. 25 minutes.
Plants: Rapidly decomposed in plants.
Soil/Environment: Non-persistent in the environment, with rapid decomposition in the atmosphere. Undergoes hydrolysis in damp media, with the formation of phosphoric acid and CO2. But G. Keller et al. say soil metabolites are dichloroethanol, dichloroacetic acid and dichloroacetaldehyde; DT50 c. 10 h. Half-lives <1 d in biologically active soils and water systems.