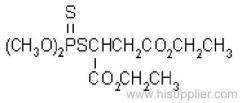|
Shanghai Skyblue Chemical Co., Ltd.
|
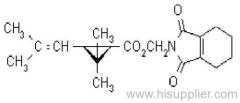
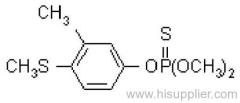
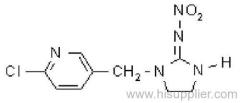
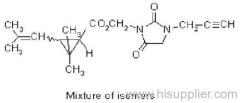
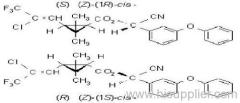
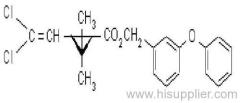
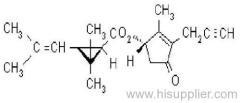
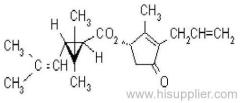
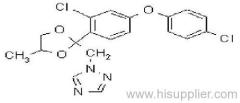
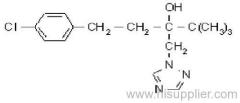
allethrin
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Control of flies, mosquitoes, ants, and other household and public health insect pests.
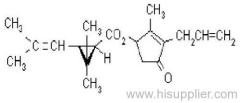
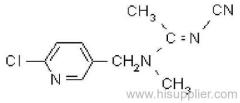
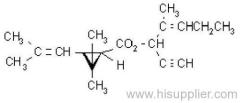
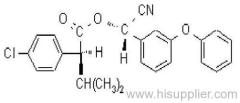
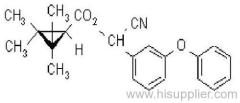
Common name: allethrin; alléthrine; d-allethrin
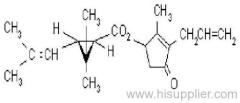
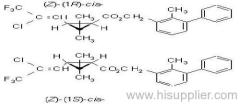
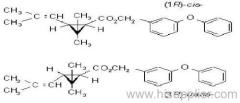
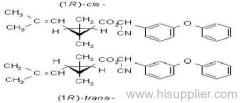
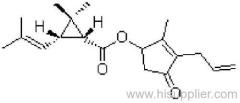
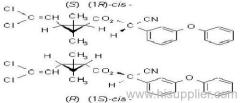
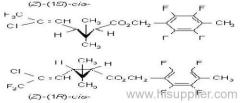
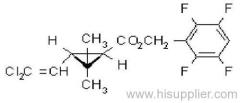
IUPAC name: (RS)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (1R,3R;1R,3S)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
Alt: (RS)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (+)-cis-trans-chrysanthemate
Roth: (RS)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (1R)-cis-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate
Chemical Abstracts name: 2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl 2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate
CAS RN: [584-79-2] (unstated stereochemistry and also used for bioallethrin)
EEC no.: 209-542-4 [for (1RS)- isomers]
PHYSICAL CHEMISTRY
Composition: d-Allethrin is 93% (1R)- isomers and 75% trans isomers; see also bioallethrin. Mol. wt.: 302.4; M.f.: C19H26O3; Form: Tech. is a pale yellow liquid. B.p.: 281.5 °C/760 mmHg. V.p.: 0.16 mPa (21 °C). KOW: logP = 4.96 (room temperature). S.g./density: 1.01 (20 °C). Solubility: Practically insoluble in water. In hexane 0.655 g/ml, methanol 72.0 ml/ml (both 20 °C). Stability: Decomposed by u.v. light. Hydrolysed in alkaline media. F.p.: 87 °C
APPLICATIONS
Mode of action: Non-systemic insecticide with contact, stomach, and respiratory action. Gives rapid knockdown. Paralyses insects before killing them.
Uses: Control of flies, mosquitoes, ants, and other household and public health insect pests. Often used in combination with piperonyl butoxide or other synergists, for control of chewing and sucking insects on ornamentals, vegetables, and other crops; for household and public health insect control; for insect control in animal houses; and as an animal ectoparasiticide.
Formulation types: AE; EC; DP; WP; Coil.
Compatibility: Incompatible with alkaline materials.
Selected tradenames: Pynamin Forte
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for male rats 2150, female rats 900 mg/kg.
Skin and eye: Acute percutaneous LD50 for male rabbits 2660, female rabbits 4390 mg/kg.
Inhalation: LC50 for rats >3875 mg/m3 air.
Toxicity class: WHO (a.i.) III; EPA (formulation) III
EC hazard: Xn; R22
ECOTOXICOLOGY
Birds: LC50 (8 d) for mallard ducks and bobwhite quail 5620 mg 'Pynamin Forte' /kg diet.
Fish: LC50 (96 h) for carp 0.134 mg/l.
ENVIRONMENTAL FATE
Animals: In mammals, following oral administration, one of the two terminal methyl groups of the chrysanthemic acid moiety is oxidised in the liver to an alcohol group, and further to a carboxyl group.
Didn't find what you're looking for?
Post Buying Lead or contact
HiSupplier Customer Service Center
for help!