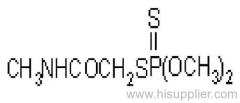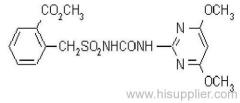|
Shanghai Skyblue Chemical Co., Ltd.
|
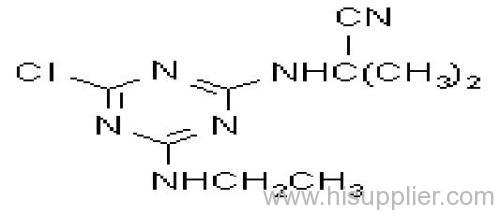
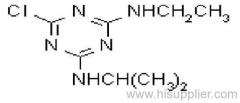
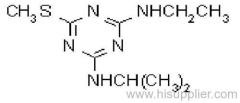
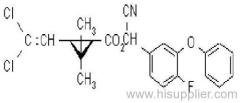
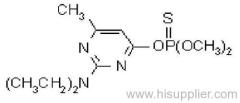
Cyanazine
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Selective systemic herbicide, absorbed by the roots (with translocation acropetally to the leaves), and also by the foliage.
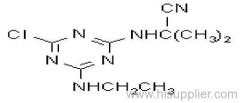
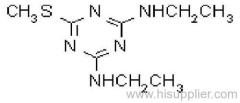
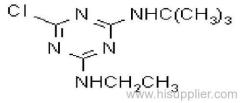
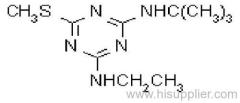
Common name: cyanazine
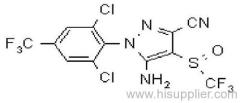
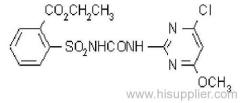
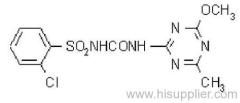
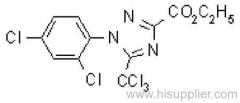
IUPAC name: 2-(4-chloro-6-ethylamino-1,3,5-triazin-2-ylamino)-2-methylpropiononitrile
Chemical Abstracts name: 2-[[4-chloro-6-(ethylamino)-1,3,5-triazin-2-yl]amino]-2-methylpropanenitrile
CAS RN: [21725-46-2]
PHYSICAL CHEMISTRY
Composition: Tech. grade 95% pure. Mol. wt.: 240.7; M.f.: C9H13ClN6 Form: Tech. cyanazine is a white crystalline solid. M.p.: 167.5-169 ºC (tech., 166.5-167 ºC). V.p.: 2x10-4 mPa (20 ºC). KOW: logP = 2.1 S.g./density: 1.29 kg/l (20 ºC). Solubility: In water 171 mg/l (25 ºC). In methylcyclohexanone, chloroform 210, acetone 195, ethanol 45, benzene, hexane 15, carbon tetrachloride <10 (all in g/l, 25 ºC). Stability: Stable to heat (1.8% decomposition after 100 h at 75 ºC), and to light. Stable in solution between pH 5 and 9, hydrolysed by strong acids and alkalis. pKa: 0.63, v. weak base
APPLICATIONS
Biochemistry: Photosynthetic electron transport inhibitor at the photosystem II receptor site. Maize tolerance of triazines is attributed to conjugation with glutathione.
Mode of action: Selective systemic herbicide, absorbed by the roots (with translocation acropetally to the leaves), and also by the foliage.
Uses: Used for general weed control (a) pre-emergence to the crop, at 1-3 kg a.i./ha, in broad beans, maize and peas; (b) post-emergence in barley and wheat during the early tillering stage, at 0.26-0.33 kg/ha in combination with a variety of other herbicides for the control of broad-leaved weeds. Other crops for which it is used include: cotton, oilseed rape, forestry, potatoes, soya beans, sugar cane.
Phytotoxicity: Selective if applied according to label recommendations.
Formulation types: DF; GR; SC; WP.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for rats 182-334, mice 380, rabbits 141 mg/kg.
Skin and eye: Acute percutaneous LD50 for rats >1200, rabbits >2000 mg/kg. Non-irritating to skin and eyes.
Inhalation: LC50 >2460 mg/m3 air, as cyanazine dust.
NOEL: (2 y) for rats 12, dogs 25 mg/kg diet.
Toxicity class: WHO (a.i.) II; EPA (formulation) II (DF, SC and WP), III (GR)
EC hazard: Xn; R22| N; R50, R53
ECOTOXICOLOGY
Birds: Acute oral LD50 for mallard ducks >2000, quail 400 mg/kg.
Fish: LC50 (48 h) for harlequin fish 10 mg/l; LC50 (96 h) for fathead minnow 16 mg/l.
Daphnia: LC50 (48 h) 42-106 mg/l.
Algae: EC50 (96 h) <0.1 mg/l.
Bees: Not toxic to bees. LD50 (topical) >100 ug/bee (tech. in acetone); (oral) >190 ug/bee (tech. as dust).
ENVIRONMENTAL FATE
Animals: In rats and dogs, following oral administration, cyanazine is rapidly metabolised and eliminated within c. 4 days.
Plants: In plants, the nitrile group is hydrolysed to a carboxylic acid group, and the chlorine atom is replaced by a hydroxy group.
Soil/Environment: Microbial degradation in soil occurs within one growth period. Metabolism is similar to that in plants. DT50 in soil c. 2 w.