|
Shanghai Skyblue Chemical Co., Ltd.
|
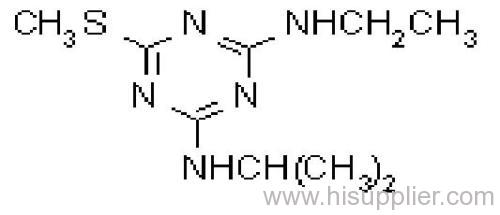
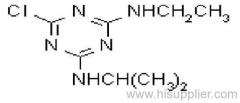
Ametryn
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Selective systemic herbicide, absorbed by the leaves and roots, with translocation acropetally in the xylem, and accumulation in the apical meristems.
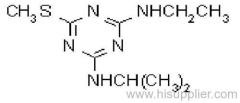
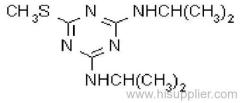
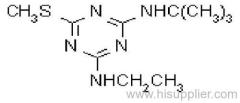
Common name: ametryn; ametryne
IUPAC name: N2-ethyl-N4-isopropyl-6-methylthio-1,3,5-triazine-2,4-diamine
Chemical Abstracts name: N-ethyl-N'-(1-methylethyl)-6-(methylthio)-1,3,5-triazine-2,4-diamine
CAS RN: [834-12-8]
PHYSICAL CHEMISTRY
Composition: 96% pure. Mol. wt.: 227.3; M.f.: C9H17N5S; Form: White powder. M.p.: 86.3-87.0 ºC; B.p.: 337 °C/98.6 kPa; V.p.: 0.365 mPa (25 ºC) (OECD 104). KOW: logP = 2.63 (25 ºC). Henry: 4.1x10-4 Pa m3 mol-1 (calc.) S.g./density: 1.18 (22 ºC). Solubility: In water 200 mg/l (20 ºC). In acetone 610, methanol 510, toluene 470, n-octanol 220, hexane 12 (all in g/l, 25 ºC). Stability: Stable in neutral, weakly acidic, and weakly alkaline media. Hydrolysed by strong acids (pH 1) and alkalis (pH 13) to the herbicidally-inactive 6-hydroxy derivative. Slowly decomposed by u.v. light. pKa: 4.1, weak base.
APPLICATIONS
Biochemistry: Photosynthetic electron transport inhibitor at the photosystem II receptor site.
Mode of action: Selective systemic herbicide, absorbed by the leaves and roots, with translocation acropetally in the xylem, and accumulation in the apical meristems.
Uses: Pre- and post-emergence control of most annual grasses and broad-leaved weeds in pineapples, sugar cane, bananas, citrus fruit, maize, cassava, coffee, tea, sisal, cocoa, oil palms, and on non-crop land. Application rates are in the range 2-4 kg/ha, except when used as a directed spray on maize. Also used as a potato haulm desiccant.
Phytotoxicity: Some sugar cane varieties show temporary chlorosis and scorching of lower leaves.
Formulation types: EC; FW; SC; WG; WP.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for rats 1160 mg tech./kg.
Skin and eye: Acute percutaneous LD50 for rabbits >2020, rats >3100 mg/kg. Not a skin or eye irritant (rabbits). Not a skin sensitiser (guinea pigs).
Inhalation: LC50 (4 h) for rats >5170 mg/m3 air.
NOEL: (2 y) for rats 50, for mice 10 ppm; (1 y) for dogs 200 ppm.
ADI: 0.015 mg/kg daily.
Toxicity class: WHO (a.i.) III; EPA (formulation) III
EC hazard: Xn; R22| N; R50, R53
ECOTOXICOLOGY
Birds: LC50 (5 d) for bobwhite quail and mallard ducks >5620 ppm.
Fish: LC50 (96 h) for rainbow trout 5, bluegill sunfish 19, channel catfish 25 mg/l.
Daphnia: LC50 (96 h) 28 mg/l.
Algae: EC50 (7 d) for Selenastrum capricornutum 0.0036 mg/l.
Other aquatic spp.: LC50 (96 h) for mysid shrimp (Mysidopsis bahia) 2.3 mg/l.
Bees: Low toxicity to bees; LD50 (oral) >100 ug/bee.
Worms: LC50 (14 d) for earthworms 166 mg/kg soil.
ENVIRONMENTAL FATE
Animals: Irrespective of the dose or the dosing regime, most is excreted within 3 to 4 days. Conjugation with glutathione and dealkylation are the main metabolic pathways.
Plants: Metabolised by tolerant plants and, to a lesser extent, by sensitive plants, to non-toxic substances by replacement of the methylthio group by a hydroxy group, and by dealkylation of the amino groups.
Soil/Environment: Loss from soil is principally by microbial degradation. Median DT50 in soil 51 d (11-120 d). Koc 300; however column leaching studies indicate ametryn does not leach significantly. Degradation in aquatic systems is caused by microbial processes, with photolysis also contributing. Adsorption to the sediment is the most efficient mechanism of elimination of ametryn from water.