|
Shanghai Skyblue Chemical Co., Ltd.
|
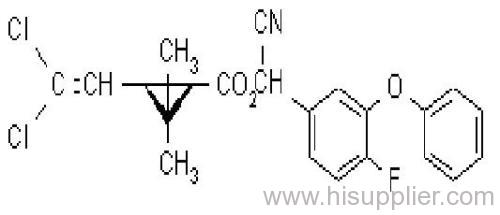
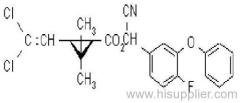
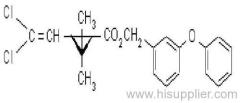
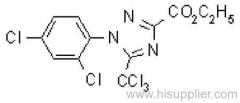
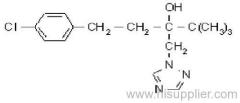
Cyfluthrin
| Place of Origin: | Shanghai, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Non-systemic insecticide with contact and stomach action. Acts on the nervous system, with rapid knockdown and long residual activity.
Common name: cyfluthrin; cyfluthrine
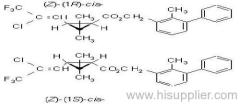
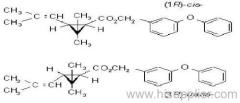
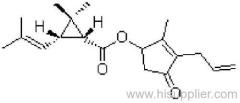
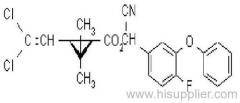
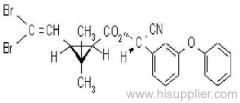
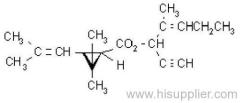
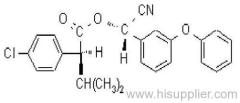
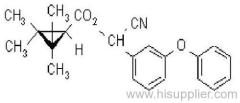
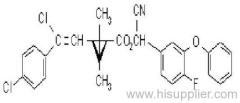
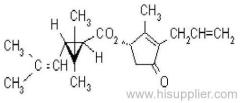
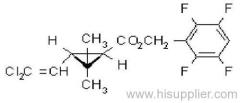
IUPAC name: (RS)-a-cyano-4-fluoro-3-phenoxybenzyl (1RS,3RS;1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Roth: (RS)-a-cyano-4-fluoro-3-phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Chemical Abstracts name: cyano(4-fluoro-3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate (unstated stereochemistry)
CAS RN: [68359-37-5]
PHYSICAL CHEMISTRY
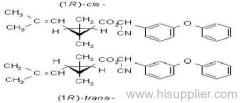
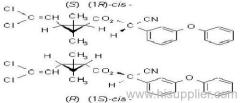
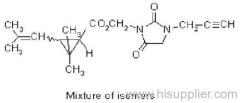
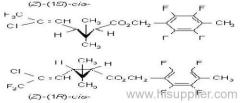
Composition: Comprises a mixture of four diastereoisomeric pairs of enantiomers:
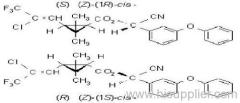
I (R)-a-cyano-4-fluoro-3-phenoxybenzyl (1R)-cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate + (S)-a, (1S)-cis-;
II (S)-a, (1R)-cis- + (R)-a, (1S)-cis-;
III (R)-a, (1R)-trans- + (S)-a, (1S)-trans-;
IV (S)-a, (1R)-trans- + (R)-a, (1S)-trans- .
Tech. grade contains: 23-27% diastereoisomer I, 17-21% diastereoisomer II, 32-36% diastereoisomer III, and 21-25% diastereoisomer IV. Mol. wt.: 434.3; M.f.: C22H18Cl2FNO3; Form: Colourless crystals; (tech. is a brown, oily, viscous mass, with crystalline parts). M.p.: (I) 64 ºC; (II) 81 ºC; (III) 65 ºC; (IV) 106 ºC; tech., c. 60 ºC. V.p.: (I) 9.6x 10-4; (II) 1.4x 10-5; (III) 2.1x10-5; (IV) 8.5x10-5 (all in mPa, 20 ºC). KOW: logP: (I) 6.0; (II) 5.9; (III) 6.0; (IV) 5.9 (all 20 °C). Henry: (I) 1.9x10-1; (II) 2.9x10-3; (III) 4.2x10-3; (IV) 1.3x10-2 (all in Pa m3 mol-1, 20 °C). S.g./density: 1.28 (20 ºC). Solubility: Diastereoisomer I: In water 2.5 (pH 3), 2.2 (pH 7) (both in g/l, 20 ºC). In dichloromethane, toluene >200, n-hexane 10-20, isopropanol 20-50 (all in g/l, 20 ºC). Diastereoisomer II: In water 2.1 (pH 3), 1.9 (pH 7) (both in g/l, 20 ºC). In dichloromethane, toluene >200, n-hexane 10-20, isopropanol 5-10 (all in g/l, 20 ºC). Diastereoisomer III: In water 3.2 (pH 3), 2.2 (pH 7) (both in g/l, 20 ºC). In dichloromethane, toluene >200, n-hexane, isopropanol 10-20 (all in g/l, 20 ºC). Diastereoisomer IV: In water 4.3 (pH 3), 2.9 (pH 7) (both in g/l, 20 ºC). In dichloromethane >200, toluene 100-200, n-hexane 1-2, isopropanol 2-5 (all in g/l, 20 ºC). Stability: Thermally stable at room temperature. In water, DT50 for diastereoisomer I: 36, 17, 7; II 117, 20, 6; III 30, 11, 3; IV 25, 11, 5 (all in days, pH 4, 7, 9 respectively, 22 ºC). F.p.: 107 ºC (tech.)
APPLICATIONS
Mode of action: Non-systemic insecticide with contact and stomach action. Acts on the nervous system, with rapid knockdown and long residual activity.
Uses: An insecticide effective against many pests, especially Lepidoptera, Coleoptera, Homoptera and Hemiptera on cereals, cotton, fruit and vegetables; also against migratory locusts and grasshoppers. For agricultural uses, applied at 15-40 g/ha. Used against Blattellidae, Culicidae and Muscidae in public health situations, stored products, domestic use and animal health. It has a rapid knockdown effect and long-lasting residual activity.
Formulation types: AE; EC; EO; ES; EW; GR; UL; WP; Oilspray.
Compatibility: Incompatible with azocyclotin.
MAMMALIAN TOXICOLOGY
Oral: Acute oral LD50 for rats c. 500 mg/kg (in xylol), c. 900 mg/kg (PEG 400), c. 20 mg/kg (water/cremophor); for dogs >100 mg/kg.
Skin and eye: Acute percutaneous LD50 (24 h) for male and female rats >5000 mg/kg. Non-irritating to skin; mildly irritating to eyes (rabbits).
Inhalation: LC50 (4 h) for male and female rats 0.5 mg/l air (aerosol).
NOEL: (2 y) for rats 50, mice 200 mg/kg diet; (1 y) for dogs 160 mg/kg diet.
ADI: 0.02 mg/kg b.w.
Toxicity class: WHO (a.i.) Ib; EPA (formulation) II
EC hazard: T+; R28| T; R23| N; R50, R53
ECOTOXICOLOGY
Birds: Acute oral LD50 for bobwhite quail >2000 mg/kg.
Fish: LC50 (96 h) for golden orfe 0.0032, rainbow trout 0.0006-0.0029, carp 0.022, bluegill sunfish 0.0015 mg/l.
Daphnia: LC50 (48 h) 0.00016-0.0027 mg/l.
Algae: ErC50 for Scenedesmus subspicatus >10 mg/l.
Bees: Toxic to honeybees.
Worms: LC50 for Eisenia foetida >1000 mg/kg dry soil.
ENVIRONMENTAL FATE
Animals: Cyfluthrin was largely and very quickly eliminated; 98% of the administered amount was eliminated after 48 h via the urine and the faeces.
Plants: Since cyfluthrin is not systemic, it penetrates only slightly into the plant tissues and is hardly translocated into other parts of the plant. The concentration is very low and can be neglected.
Soil/Environment: Degradation in different soils is rapid. The leaching behaviour can be classified as immobile. The metabolites of cyfluthrin are subject to further microbial degradation to the point of mineralisation to CO2.